Chelating Resin
Instead of ion-exchange groups, a resin with a functional group that forms a chelate (complex) with a metal ion captures the metal ion by forming a chelate, so it is called a chelate resin.
As chelate-forming groups, those containing two or more electron donating elements such as N, S, O, and P are used, and the same concept as general chelating agents can be achieved. For example NO system, SN-based, NN system, but there are types such as OO system, iminodiacetic acid-type [-N(CH2COO-)2] and polyamine type [-NH(CH2CH2NH)n·H] Is famous.
Diaion chelate resins are (1) for general metals, iminodiacetic acid type Diaion CR11, (2) polyamine type that hardly captures alkali metals (Na, K, etc.) and alkaline earth metals (Ca, Mg, etc.) There are two types, CRB03 and CRB05 of methyl glucamine type that selectively captures boric acid, etc., and the chemical structure and chelate formation structure of the resin are shown below. In the following structure, R indicates cross-linked polystyrene with divinylbenzene.
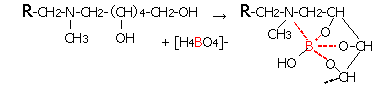
Chelation Mechanism of DIAION CR11
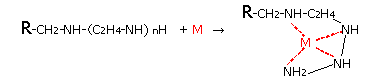
Chelation Mechanism of DIAION CR20
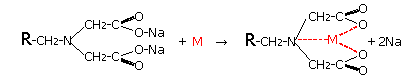
Chelation Mechanism of DIAION CRB03 and DIAON CRB05
| Product Name | CR11 | CR20 | CRB03 | CRB05 |
| Construction |  |  |  | |
| Appearance index | 95 or more | |||
| Apparent density (g / L-R) | 745 | 745 | 745 | 745 |
| Acid adsorption capacity (meq / mL – R) | – | – | 0.7 or more | 0.7 or more |
| Cu adsorption capacity (m-mol/mL-R) | 0.5 or more | 0.5 or more | – | – |
| moisture(%) | 55-65 | 55-65 | 55-65 | 55-65 |
| Particle size distribution | 1,180 μm to 5% less than 355 μm 2% or less | 1,180 μm to 5% less than 355 μm 2% or less | 1,180 μm to 5% less than 355 μm 2% or less | 1,180 μm to 5% less than 355 μm 2% or less |
| Effective diameter (mm) | 0.40 or more | 0.35 to 0.55 | 0.35 to 0.55 | |
| Uniformity coefficient | 1.6 or less | |||
| Volume change rate | 1.25 (Na+/H+) | 1.25 (Na+/H+) | – | – |
| Service temperature (°C) | 80 or less (H type) | 100 or less | 100 or less (free form) | |
| 120 or less (Na type) | (OH type) | |||
| Use | Heavy metal removal chemical solution purification of saltwater purification wastewater | Waste water treatment chemical solution purification | Low concentration boric acid removal | Borate ion removal |
Chelate resins are characterized by much greater selectivity to specific metal ions than ion exchange resins. For example, DIAION CR11 can remove several ppm of Ca, Mg, and Sr in saturated saline to a few ppb or less. can. In ion exchange with ion exchange resin, selectivity for these metal ions is higher than Na, but there is not much difference in selectivity. Mg and Sr cannot be captured.
Acids such as hydrochloric acid and sulfuric acid are usually used for desorption of adsorbed metal ions. This is because the stability of metal chelates is low at low pH, and it takes advantage of the property of chelate degradation. However, in the case of DIAION CR11 and DIAION CR20, etc., selectivity is very strong for trivalent ions and Hg, and a large amount of regenerant is required for regeneration, and sometimes regeneration is difficult. Also, some metal ions form complex salts in hydrochloric acid solution. If hydrochloric acid is used as a regenerant, it may not be regenerated. In such cases, it is effective to use sulfuric acid as a regenerant.

